Laboratory Risk Management
Solutions
Effective risk management in the lab ensures continuous, high-quality performance while increasing efficiencies, minimizing human error, and reducing system downtime.

Problem + Solutions
How Can Clinical Laboratory Automation Improve Your Compliance?
The demands of lab quality control and compliance require extensive time and resources to keep up with. Add to that the unexpected occurrences of lab downtime and you have a potential barrage of daily threats to derail your lab operations and performance. Our innovative risk management solutions bring ease to these challenges through streamlined automation for lab quality assurance you can count on.
Automate QC through a single platform
Decrease labor hours and improve patient care through automated QC programs connected to Instrument Manager lab workflow software.
Minimize errors and disruptions
Simplify College of American Pathologists (CAP) compliance reporting with automated, electronic proficiency test result delivery.
Optimize lab uptime
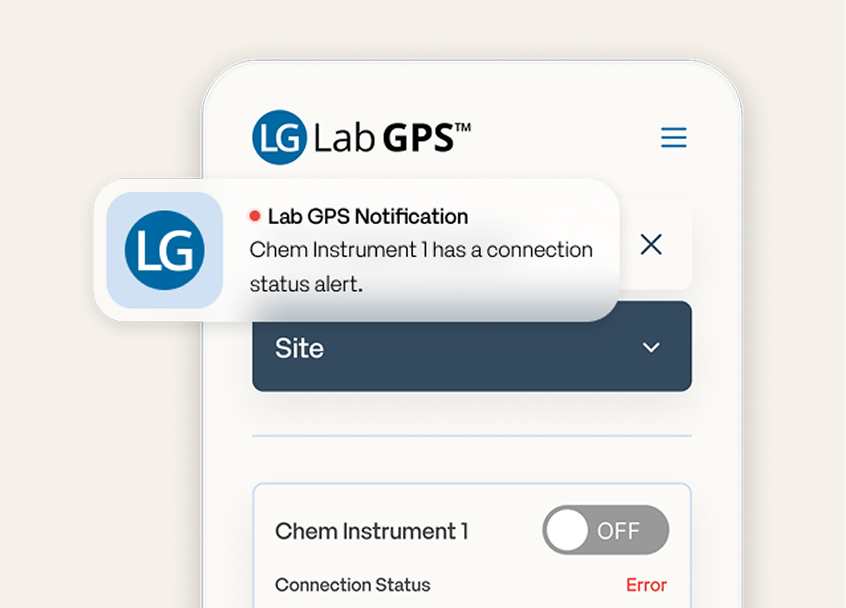
Lab GPS™ gives laboratorians all-in-one connectivity monitoring, alerts, and troubleshooting to reduce unplanned downtime.

Modules
Laboratory Workflow Management
Effective risk management in the lab ensures continuous, high-quality performance while increasing efficiencies, minimizing human error, and reducing system downtime.
Moving Averages & Moving Medians
Catch and correct QC issues in real-time by proactively monitoring and detecting systematic changes in instrument performance between QC cycles.
Identify problematic results before they reach patients and practitioners, protecting your credibility. With less result rework, you can focus on ensuring the most timely delivery of results to patients who need them.

Daily & Peer QC Integration
Streamline quality control workflows via automated QC assessments with Bio-Rad QC OnCall™, Unity Real Time®, and other QC programs.
Daily & Peer QC Integration from Instrument Manager (IM) lets your lab maximize the benefit of 3rd party lab QC programs with integrated result delivery, optimized lab workflow, and simplified peer comparison.

Lab Proficiency Reporting
Simplify lab proficiency testing and reporting to the College of American Pathologists (CAP) or American Proficiency Institute (API).
Save time and reduce reporting errors with automated, electronic proficiency test result delivery. Eliminate the need to manually enter proficiency test results on paper or online, significantly reducing clerical errors.

EP Evaluator®
Accelerate and improve the accuracy of instrument validation. Perform calculations for 100+ studies simultaneously and simplify instrument-to-instrument comparison.
EP Evaluator also provides clear, concise, ‘inspector-ready’ reports meeting CLIA, CAP, The Joint Commission, and COFRAC requirements.

Lab GPS™
Optimize lab uptime with anytime, anywhere connectivity management through Lab GPS.
Lab GPS addresses the problem of lab downtime by empowering laboratorians with all-in-one connectivity monitoring, notifications, and troubleshooting.

High Availability & Disaster Recovery
Avoid the risk of lab downtime with automatic failover and offsite disaster recovery.
Our High Availability and Disaster Recovery solutions provide automatic failover for uninterrupted operations, keeping your lab on its feet in the event of an unplanned outage.

Lab QC
Simplify your lab’s QC processes with an integrated QC solution
The demands on your lab are increasingly complex. Lab QC provides an integrated, end-to-end QC program to simplify your QC protocols and help protect patient outcomes.
*Lab QC is not available in North America*

Ready To Learn More?
Hear from Our Clients
“By using EP Evaluator, we have experienced a huge improvement in productivity. Our 6-month instrument-to-instrument comparison report data entry time went from probably 10 hours to 3 seconds.”
Products
Do More with Less
Streamline lab operations with scalable, vendor-neutral products that boost efficiency, reduce costs, and maximize performance.
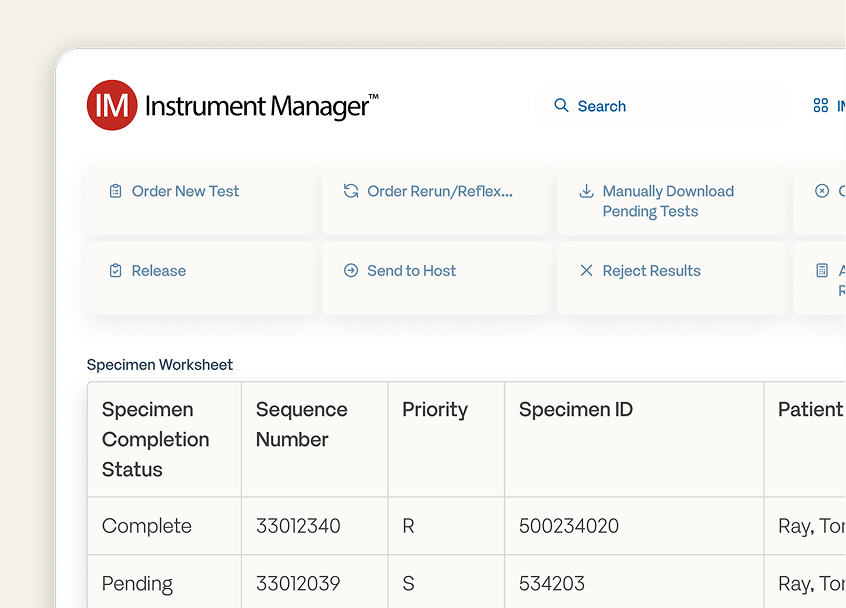
Instrument ManagerTM
Achieve your lab’s full potential with the industry’s most-used middleware platform for unrestricted connectivity, unlimited scalability, and centralized workflow management.
Featured Resources